Abstract
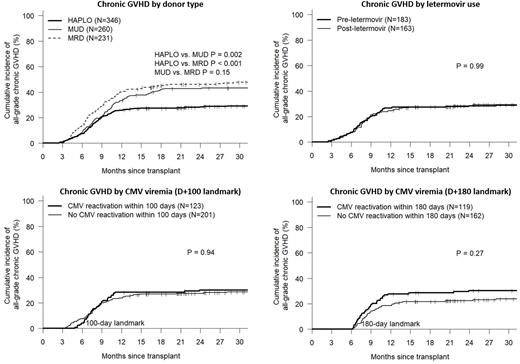
A recent CIBMTR analysis describing the increased incidence of CMV infection following post-transplant cyclophosphamide (PTCy) (Goldsmith et al. Blood 2021, 137(23):3291-3305) also demonstrated higher chronic graft-versus-host disease (cGVHD) rates in PTCy recipients following CMV reactivation. The authors suggested that CMV reactivation may potentially negate the known protective effect of PTCy on cGVHD incidence. However, such an analysis can be confounded by the close association of cGVHD and CMV reactivation making cause-and-effect associations challenging. In the hopes of better understanding this association, we analyzed 346 consecutive PTCy-based haploidentical donor transplants (HAPLO) performed at a single institution between Jan 2012 to June 2021. We first evaluated the effect of CMV reactivation on cGVHD incidence utilizing both a D+100 and D+180 landmark analysis. Secondly, we analyzed the effect of routine CMV prophylaxis with letermovir, which was initiated in all HAPLO recipients starting Jan 2018, on cGVHD incidence. Given that letermovir reduces the risk of CMV reactivation, we hypothesized that pre-letermovir cGVHD rates in HAPLO recipients should be higher if CMV reactivation was a true causative risk factor for cGVHD. Baseline characteristics of HAPLO recipients included median age 53 (range, 19-80), 58% male, 52% non-Hispanic white , HCT-CI ≥3 in 60% and CMV-seropositive in 76%. Myeloablative conditioning was used in 42% of transplants. CMV viremia requiring preemptive therapy occurred in 155 patients, with a median time to reactivation of 40 days (range 1-645) with 86% of reactivations occurring before day +100. Patients receiving letermovir prophylaxis had lower CI of CMV viremia when measured at day +100 (17% vs. 57%) and day +180 (26% vs. 57%) respectively compared to patients not receiving letermovir. There was no difference in baseline characteristics between those receiving and not receiving letermovir prophylaxis. Compared with contemporaneously treated MRD and MUD recipients receiving conventional GVHD prophylaxis, the CI of cGVHD was lower following PTCy-based HAPLO (28% vs. 46% and 43% respectively). The CI of cGVHD did not differ in HAPLO recipients with or without CMV reactivation, whether using a day +100 or day +180 landmark analysis. Similarly, there was no difference in cGVHD incidence in HAPLO recipients that received CMV prophylaxis with letermovir vs. those that did not (see figure). A subsequent analysis evaluating the CI of moderate-to-severe grade cGVHD showed similar findings with no effect of either CMV reactivation or letermovir use. Cox multi-variable analysis evaluating CMV reactivation as a time-dependent covariate confirmed the lack of effect of CMV viremia on cGVHD incidence (HR 1.1, p=0.65). In summary, our large single-center analysis of PTCy-based HAPLO recipients did not confirm the prior CIBMTR findings of increased cGVHD incidence following CMV reactivation. Furthermore, cGVHD incidence was not influenced by letermovir prophylaxis despite significantly lower rates of CMV reactivation. We speculate that the previously published association of higher cGVHD rates with CMV reactivation after PTCy-based transplants may represent an epiphenomenon, whereby CMV reactivation in some patients may predate the clinical diagnosis of cGVHD.
Disclosures
Solh:ADC Therapeutics: Research Funding; Partner Therapeutics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal